Abstract
Introduction
Localized radiotherapy (RT) is the standard treatment in solitary plasmocytoma (SP). Before the implementation of the current guidelines also "single lesion multiple myeloma" (SLMM) patients who were diagnosed with one single either lytic or non-lytic bone lesion were classified as Durie/Salmon stage I and oftentimes treated with radiotherapy or surgery followed by a phase of thorough surveillance even if they showed signs of systemic disease activity without advanced end organ damage. However, according to the recently updated IMWG criteria defining multiple myeloma (MM), patients now are recommended to be treated with systemic therapy if - among others - at least one osteolytic lesion in conventional skeletal survey or computed tomography (CT) or more than one focal bone marrow lesion in magnetic resonance imaging (MRI) is detected, whereas SLMM patients with only one lesion in MRI still receive no systemic therapy. Clinical experience has revealed that some of the SLMM patients treated with local therapy only had a rather long progression free and therefore treatment free interval until systemic intervention was necessary. Therefore we retrospectively studied a cohort of patients whom we gave the contradicting diagnosis of a "SLMM" to evaluate which therapeutic approach would be most beneficial.
Methods
From our patient database we identified 89 SLMM patients who presented at the University Hospital of Heidelberg with a histologically verified, newly diagnosed single lesion and a plasma cell infiltration of ≥10% between 05/1996 and 03/2017. We included both, patients with lytic and non-lytic single lesions since both could have been treated upfront with either local or systemic therapy depending on the respective classification system in use and clinicians choice. Median age was 57 years (range 40-77 years). Single lesions were identified upon diagnosis by whole body imaging with single lesions detected by CT scan in 57 of the patients, by MRI scan in 88 patients and/or by skeletal survey in 15 patients. We analyzed the impact of clinical characteristics and treatment strategy on progression free survival (PFS) and overall survival (OS). Kaplan Meier plots and corresponding log-rank test p-values were generated. Cox regression was used to estimate the hazard ratio.
Results and Discussion
A total of 61 of the 89 SLMM patients had symptomatic myeloma according to the updated IMWG diagnosis criteria, in 57 of these symptomatic patients the single lesion was detected by CT scan and/ or skeletal survey, in 4 of the patients the lesion was identified by skeletal survey only. According to IMWG guidelines, 28 of the 89 SLMM patients were asymptomatic and presented with a solitary lesion in MRI only. According to the Salmon Durie classification 70 patients classified for stage I, 9 patients for stage II and 10 patients for stage III.
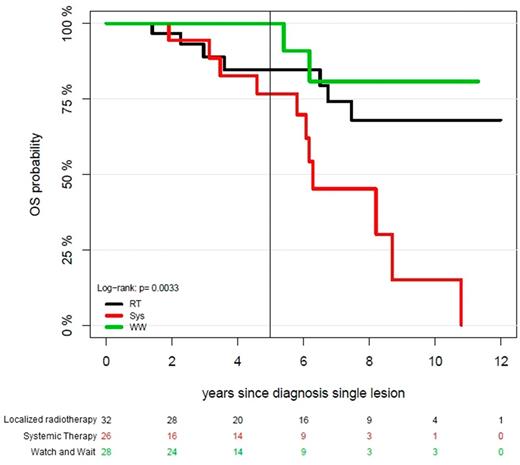
Of the whole cohort 26 (29.2%) patients were treated with chemotherapy upfront, 32 (35.9%) patients received RT, for 28 patients a "watch and wait" strategy was chosen (31.5%). Patients with a "watch and wait" strategy or RT as initial treatment showed no significant difference in PFS compared to patients who received systemic therapy upfront (p=0.14). Interestingly OS of patients with surgery only or RT was superior to those with upfront systemic treatment (p=0.033) with a 5 year OS of 100% for "watch and wait", 85% for patients receiving local therapy and 77% for patients treated systemically (median OS not reached), (Fig. 1). Of note, the set of all patients who had received no systemic therapy had a low disease burden with ISS-I and modest bone marrow plasmocytosis (median 22.0%), while the systemically treated cohort comprised of all three ISS stages and a higher bone marrow plasma cell infiltration rate (38.8%).
Even so, SLMM patients with low disease burden and no initial systemic treatment progressed within 1.5-3 years to a higher stage MM requiring systemic therapy, they still showed excellent long-term OS. This suggests that the initial choice of local therapy may prolong therapy free intervals for SLMM patients and thereby improve quality of life without negative effect on OS.
Conclusion
Our data suggests that there might be a patient cohort among MM patients whose survival is not impaired by delayed systemic treatment. Still this distinct SLMM group has to be better characterized clinically as well as biologically avoiding over- and more importantly under-treatment.
Goldschmidt: Morphosys: Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Onyx: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Millenium: Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Chugai: Consultancy, Honoraria, Research Funding, Speakers Bureau. Hillengass: BMS: Honoraria; honoraria from Amgen, BMS, Celgene: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal